Empirical Formula of Ascorbic Acid
Vitamin C is known chemically by the name ascorbic acid. So its often called the simplest formula so.
In 100 g of ascorbic acid we will have 4092 g C 458 g H and 5450 g O.
. This is the empirical formula for Ascorbic acid. So the empirical formula of ascorbic acid will be. What is the empirical formula of ascorbic acid.
The elemental mass percent composition of ascorbic acid vitamin C is 4092 C 458 H and 5450 0. The whole-number ratio gives us the subscripts for the empirical formula. What is the empirical of ascorbic acid.
Applications Products Services Support. Determine the empirical formula of lactic acid given that combustion of a 100-g sample produces 147 g CO and 600 g Hao. 25 26 Vitamin C is known chemically by the name ascorbic acid.
For example in Sample Exercise 312 we. We can then divide by the smallest value and round. The whole-number ratio gives us the subscripts for the empirical formula.
What is the empirical molar mass of ascorbic. To get the empirical in this case we divide by the smallest value. VIDEO ANSWERThis question has start with our definition of empirical formula so this is the smallest the whole number ratio for a compound.
Empirical weight 3 x atomic wt. To determine the empirical formula of ascorbic acid you have to perform a qualitative analysis and determine the percent by mass of each element present in the molecule. Ascorbic acid contains carbon hydrogen and oxygen.
Ascorbie acid vitamin C a white crystalline solid that is present in fruits and vegetables curves scury and may help. In short Empirical formula eqrm fracMolecularFormulaHCF eq HFC is the Highest Common Factor. The empirical formula is C3H6O2 Empirical formulan molecular mass C3H8O2n 228276 12x3 816x2n 228276.
The multiple is found by comparing the formula weight of the empirical formula with the molecular weight. If we multiplied our empirical formula by 2 then the molecular. C 4 x atomic wt H 3 x atomic weight O.
Simplest or empirical formula. Thus it would appear that our empirical formula is essentially one half the mass of the actual molecular mass. The empirical formula and the molecular formula of the ascorbic acid should be identified when the combustion of 281g ascorbic acid produces 115g of H 2 O and 421g of CO 2.
Since nCnHnOis 3 mol C4 mol H3. C 6 H 8 O 6 C 3 H 4 O 3 textC_cancel6textH_cancel8textO_cancel6 rightarrow. Given the percentage mass we can determine the mass of each element in 100g of ascorbic acid and calculate their number of moles.
Mol O the empirical formula is C3H4O32 Apr 2022. Find ascorbic acid and related products for scientific research at Merck. The empirical formula of ascorbic acid can be described as the simplified form of the chemical formula of ascorbic acid where the ratio of each atom is still observed.
Determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450 oxygen. Determine the empirical formula of ascorbic acid. Given that the pseudoformula is c3407h453o3406.
In 100 g of ascorbic acid we will have 4092 g C 458 g H and 5450 g O. Empirical weight 3 x 12 4 x 1 3 x. Determine the empirical formula of ascorbic acid if it is composed of 40925 carbon 4586.
Express your answer as a chemical formula. Multiplier molar mass empirical weight 17612g empirical weight g.

Pin On Typography Design Handwritten

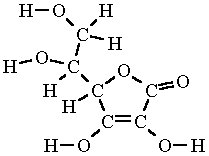
Ascorbic Acid Formula Structure

General Chemistry I Solving For An Empirical Formula Youtube

0 Response to "Empirical Formula of Ascorbic Acid"
Post a Comment